Selected Articles from the
October 2000 Odyssey
Editor: Terry Hancock
Many Worlds!
The Outer Solar system is the land of the Ice Giants: worlds so large they dwarf our own, and leave us with little to use for comparison. Moon systems which are like solar systems themselves, but in miniature. Jupiter's retinue includes four moons that might as well be called planets in their own right. Then there's Saturn's moon Titan, and Neptune's Triton.
With the Voyager missions, we were stunned by the diversity we found in what we had fully expected to be nothing but boring balls of ice and rock, perhaps a little "like our own Moon," but colder.
After Voyager, we felt a false sense of security: that we had now “explored” these worlds and that they would have few surprises left for us. Nevertheless, more recent finding have shown us that we have but scratched the surface....
This is our theme for the newsletter this month!
The Needs of the Many...
By Robert Gounley
Success can have unexpected consequences, especially in space exploration.
Five years ago, the Galileo Orbiter delivered an atmospheric probe deep into Jupiter's clouds; from it we gained vital clues about how our solar system was born. The Orbiter went on to make over twenty close fly-bys of the four major moons of Jupiter -- Io, Europa, Ganymede, and Callisto. From these encounters, we know parts of these worlds as well as we know some of the most remote sections of our own planet. It did all this while mapping Jupiter's magnetic fields and the belts of atomic particles trapped within it. Although the electronics occasionally protested, the spacecraft calmly absorbed radiation doses capable of killing a human passenger hundreds of times over.
Today, the spacecraft is battleworn, but working. With care, it could continue returning useful data until its propellant tanks run dry, and probably well beyond. However, the Galileo Orbiter will not be allowed to fade away slowly like most interplanetary explorers before it. Instead, it will be directed to end itself by entering Jupiter's atmosphere. Ironically, the main reason for this drastic measure is a consequence of its greatest success -- the mysteries revealed at Europa.

|
| Jupiter and moons. NASA photograph. |
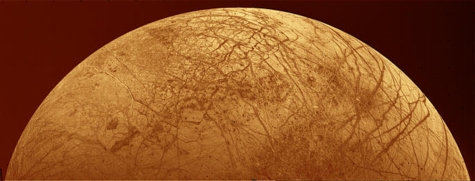
Smaller than Earth's own moon, Europa is the junior member Jupiter's major satellite. It's is an icy, billiard ball of a world. Beyond shallow, gravel-filled grooves that make the moon look like finely cracked porcelain, there are very few large features. Compared to its more photogenic neighbors, Europa seems pretty dull stuff.
However, this absence of features drew scientists' attention. Early in our solar system's formation, meteors pummeled the new worlds. Cold inert worlds, like our Moon or Callisto, kept the scars of their youth and steadily added new craters to their aged faces. Active worlds, like Earth with its atmosphere and oceans, or Io with its volcanoes, undergo regular makeovers that render ancient meteor craters almost invisible.
Europa is somewhere in between. A few small craters are visible, but none of the really big ones that are telltale signs of early impacts. To look so blemish-free suggests that Europa's surface must have been liquid, or at least slushy, for sometime after the great meteor bombardment billions of years ago. One could imagine Europa as a remote ocean world, slowly freezing as life began on a much warmer Earth.
There the speculation might have ended if Galileo had not come by to explore. When earthbound scientists saw high-resolution pictures of Europa, they were surprised to see evidence that it did not freeze evenly. In places, blocks of ice stand above the surface in interlocking patterns, like icebergs broken off a glacier and then frozen in place.
Still more intriguing were pictures showing a very smooth spot in the middle of a much rougher field -- signs that something had warmed the area before refreezing?
Europa has not been idle these past billion years. From time to time some source of internal heat melted the ice in ways visible on the surface. If so, could there have been an ocean beneath the ice sometime in the geologically recent past? (Recent, for a geologist, is within the past several million years.) More intriguing, geothermal processes, like the one around deep-sea vents on Earth, could sustain life without sunlight provided there was liquid water and the right assortment of carbon compounds. Could Europe have been touched by life?
Scientists received an even greater jolt when sensors on the Galileo Orbiter detected a weak magnetic field around Europe. The way it mingles with Jupiter's magnetic field showed that it wasn?t due to a ferromagnetic core like Earth's. Rather, it behaves as if Europa has an electrically conductive surface. Ice and stone conduct electricity poorly, but salt water does it well.
This is far short of proof. Still, although amazing to imagine, an ocean below kilometers of ice best fits the available evidence. This hypothesis is inspiring many ideas to explore beneath Europe's ice. Someday, unmanned probes (Jovan radiation being severely unkind to humans) may drill kilometers down to see what's below. To make this meaningful, everything manmade that reaches Europa must be absolutely sterile. If not, we may find ourselves inappropriately surprised to find bacteria on Europe which bear an astonishing resemblance to ones from Earth.
|
|
| Europa. NASA photograph. |
This brings us back to Galileo. It has been touring the Jovan satellites by using close swingbys to deflect its path and line up new encounters. This still requires a little propellant for its thrusters, so the tanks are beginning to run dry. By 2004 or so, the gas gage will read empty and Galileo will become a free-floater, unable to change course. Chances are, it will orbit around Jupiter for millions of years without incident. Galileo, however, was not sterilized during its assembly. A few traces of terrestrial life might yet endure in some of the more heavily shielded electronics bays. There is the possibility, difficult to quantify that someday Galileo will collide with Europa and leave traces of terrestrial life there.
However improbable this may be, mission planners will not leave Galileo as a derelict spacecraft. Instead, Galileo will use its the last of its propellant to steer itself onto a collision course with Jupiter. There, it will burn up in the atmosphere, joining the remains of the Galileo Probe it delivered there eight years earlier.
It is a hero's death of sorts. I'm reminded of the movie Star Trek III: The Search for Spock, where the Enterprise crew scuttled their ship in order to rescue Mr. Spock. Silently, they look skyward as their vessel blows apart. Their faces show more than losing their ride home. A bond was broken. They were as emotionally joined to their ship as any human being (or Vulcan) can be to a physical object.
At the appointed date, I hope to join the Galileo flight team, with whom I've shared so many remarkable experiences, to watch as Galileo's last signals come to Earth and are heard no more.
Later, it will be time to look up at Jupiter in the night sky ... and remember.
Mysteries of the Deep
By Terry Hancock
Descending into the depths, you hear the metal strain as the water presses in around you. You are now deeper than any ocean on Earth, under kilometers of ice and kilometers more of salt water. The pressure is not so extreme because of the low gravity, but it is a long way back up to space and the darkness is oppressive in the claustrophobic confines of the submersible. Finally, below you, you see a faint glow, invisible but for the impenetrable darkness engulfing you -- bioluminescence? But a shadow engulfs you again and your ultrasonics indicate something large moving up from the depths...
Talking about life on Europa leads us to fantasies of this type, but how realistic is it really? Is Europa really the “place to look”?
I find it ironic that the wide acceptance of the possibility of life on Europa followed Clarke's proposal of the idea in 2010: Odyssey Two, just as wide acceptance of the idea of life on Mars followed Wells's War of the Worlds. It seems we have a powerful tendency to be swayed by our fantasies.
Exobiology -- still mocked as the only science without a subject -- has been plagued more than any other field by this kind of observer bias, and it extends beyond the theoretical field into politics and popular culture. Nowhere does real and pseudo- science get more hopelessly entangled! Yet real exploration is possible, though without the harshest experimental tests, discoveries may be revealed as meaningless false positives.
But, we must also remember not to limit our search excessively, creating false negatives by simply not looking. The real lesson of Europa is that we have not scratched the surface of the outer solar system. There are mysteries that we still don't even know the right questions for, let alone the right answers!
Life as we know it?
There is a popular (and understandable) bias for life “as we know it” -- that is, based on the same chemistry as we. The argument goes something like this: only carbon chemistry (and in particular, nucleic acid or protein chemistry) has sufficient stability and pliability under “reasonable conditions” to provide the chemistry for life. Then, strangely, we are told that, furthermore, we must look for temperatures and pressures close to those we find on Earth, because only at those temperatures and pressures are “life molecules” sufficiently stable and pliable. Too cold, and reactions won't go. Too hot, and the molecules fall apart.
See any problems?
Yes! This is a circular argument: we need standard temperature and pressure to make a chemistry work, that we only picked because it works at standard temperature and pressure. All we really know is that life is possible with carbon-based nucleic acids and proteins in water -- we don't have strong evidence for this being the only type of life, since our argument is purely a heuristic explanation for life as we know it. So are there counterexamples? Well, there are some possibilities.
It seems to me that the more likely situation is that life can be made of a lot of different types of stuff, so long as certain basic properties are present, for which I'd offer the following guesses: 1) some kind of liquid millieu, which encourages chemical reactions. 2) some form of codable polymer -- that is, a polymer in which the monomers are not identical, but can have substitutions which affect the behavior of the resulting molecule, and 3) some source of free energy that can drive the reactions. We also need a method that could have driven the genesis and evolution of life -- it's not enough that modern, well-enough-adapted life forms could survive there, but also that there was a cradle for early evolution, like the primordial seas and template clays on Earth that may have led to early RNA-cycle life forms.
Titan?
In the much lower temperature and highly reducing environment on Titan, for example, we find semi-stable polyacetylene and cyanogen polymers (called “tholins”). The monomer, acetylene (or ethyne), is a popular welding gas, because it has such a large amount of energy stored in it. This stuff contains so much energy, that some people have seriously suggested that the Huygens probe may trigger an explosion on impact, thus destroying itself. What an image that is!
Research on these materials is quite hard to do on Earth, and what we mainly know is that they can be quite complex. It's clear that they form large polymers, and it has been suggested that they may exhibit controlled folding behaviors (just as proteins do in water on Earth). This may cause them to be suspended for long periods of time in the (hypothetical) methane or ethane oceans on Titan, even if they don't float or dissolve (methane being a much poorer solvent than water). And once they do sink down into the mire under the ocean, what then, when they're in close contact with others of their kind? Will they interact? Polymerize? Replicate?
We've had about twenty years to experiment with these compounds, while nature has been at it for over four billion years on Titan. It would be the height of hubris to think we know what's possible in this environment. So we have polymers, a liquid millieu, and a source of free energy in the solar UV flux that drives the “tholin” production in the upper atmosphere. Yet, we are assured, that Titan is simply too cold for life to evolve there.
Yes, and Triton is way too cold for geysers, too!
Io?
Similar arguments can be made for other “hostile” locales. Io has a liquid millieu just below the surface in the form of liquid sulfur, and sulfur also forms polymers under certain conditions. But this is less promising, since sulfur polymers don't allow for coding -- they're all just -S-S- bonds, and the structures tend to be unstable. Large scale organization may not be feasible.
On the other hand, at high temperatures, in combination with phosphorus and silicon, sulfur can have some very interesting chemistry indeed. Is it enough? This is unknown, but it's a mistake to be too closed minded about such things. By now we should have learned to expect the unexpected.
Europa?
Here we're in more familiar territory: we have the liquid millieu in the form of water, and the temperatures and pressures are consistent with those we are familiar with. There are many polymers available, including, but not limited to the nucleic acids and proteins we are familiar with.
The most promising energy source is gravity. The same tidal forces that keep the ice melted, may cause volcanism (on a smaller scale than Io), which could bring fresh oxidizing chemicals (such as sulfur, as on Io) to the sea floor, enriching it for chemotrophs at the boundary. This is exactly what happens in deep sea vent communities on Earth.
The only problem with this argument is that we don't really have evidence for these vents. They are merely plausible given the situation on Io, and the (rather faint) evidence for a liquid, salty ocean on Europa. But it certainly opens the door to the possibility, since this would make Europa's oceans the most Earth-like environment we've yet discovered.
What would it mean if we did find life on Europa? The first question would be of genesis -- was it a separate genesis or the same as ours? If we find life that is “life as we know it” -- that is, based on DNA and protein, then it's probably a safe bet that there was a common genesis. Similarity between the genetic coding, would of course, be utterly convincing for that hypothesis, but even the mere use of the same chemical basis would highly imply it. Otherwise, we would be faced with the conclusion that there is only one path to the large-scale organization we call “life”. That conclusion on the other hand, would be pretty stunning as well. What do we make of a universe that only has one custom-tailored method of making life-forms? Is this the signature of a creator? As Carl Sagan liked to say, “Well ... Maybe.”
What's the point to all of this?
Well, I don't mean to imply that we can expect to find life in all these places, but rather to draw our certainties about what “can't be” into some doubt. There's too much out there, and too little facts to go on, for us to think we have a handle on these problems. There are entire worlds out there unexplored, and each one of them is large and complex in itself, just like our own.
Unless the Huygens probe should image giant sedentary chemotrophs soaking up the tholin precipitation, or a Europa drill probe should pull up the local equivalent of algae, we are unlikely to resolve these questions soon, but we will acquire more the facts that we need to pursue the questions: We can prove (or disprove) the existence of a water ocean on Europa or a methane ocean on Titan. We can know in detail what the conditions are, and we can therefore simulate what sorts of chemistry are possible there.
The business of exploring the Solar System has only just begun. We need depth as well as breadth if we are to really understand the worlds around us!
For the references on this article are online.
Copyright © 1998-2003 Organization for the Advancement of Space Industrialization and Settlement. All Rights Reserved.